Understanding Low Migration Inks for Rigid Container Packaging
Low migration inks play a critical role in ensuring consumer safety and regulatory compliance in the food and beverage packaging industry. They not only protect health but also uphold brand integrity, making them essential for any company focused on quality and safety in packaging.
What are Low Migration Inks
Low migration inks are specifically formulated to minimize the risk of ink components migrating into the food or beverage contained within a package. Migration occurs when substances from the ink, such as solvents, additives, or pigments, move through the packaging material and come into contact with the product. This can lead to potential contamination and adverse health effects.
These inks are designed to be less likely to migrate, meeting stringent safety and regulatory requirements. They are particularly important in rigid packaging applications for food and beverages where the printing ink is printed on the non-food contact side of the primary package.
| How Ink Migration Can Occur From Printing Three common paths where migration can occur: | |
|---|---|
| Physical Migration | Penetration migration from printed side through substrate onto unprinted side 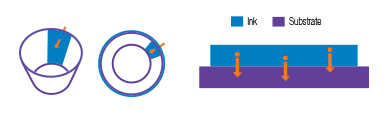 |
Set-off migration from printed side to unprinted side of another sheet in a stack or roll 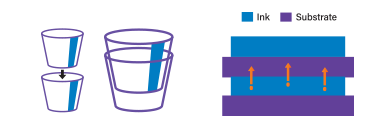 | |
| Gas Phase Migration | Evaporation or condensation migration, either due to the evaporation of volatile materials by heating (e.g. cooking, baking, or boiling frozen products in their original packaging) or the condensation through steam distillation 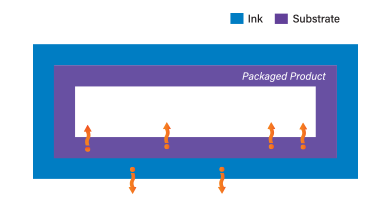 |
Why Low Migration Inks Matter
- Regulatory Compliance: Many regions have strict regulations governing packaging materials, particularly for food and beverages. For example, in the European Union, the Regulation (EC) No 1935/2004 requires that materials and articles intended to come into contact with food must not transfer their constituents to the food in quantities that could endanger human health. Low migration inks help meet these regulations, ensuring that packaging is compliant with safety standards.
- Consumer Safety: The primary concern with migration is the potential risk to consumer health. Low migration inks reduce this risk by minimizing the chance that harmful substances will leach into food or drink products. This not only protects consumers but also enhances the reputation of the brand by demonstrating a commitment to safety and quality.
- Product Integrity: Migration can also affect the quality and taste of the product inside the package. Low migration inks help maintain the integrity of the food or beverage by preventing any unwanted flavors or odors from contaminating the product.
DOWNLOAD
Learn more about low migration inks and coatings for packaging applications.
Download Brochure
When to Use Low Migration Inks
- Food and Beverage Packaging: This is the most obvious application. If your rigid container packaging is intended for any consumable product, using low migration inks is essential. Whether you're packaging yogurt, ice cream, or any other food items, ensuring that your inks are low migration is crucial for meeting safety standards and protecting consumer health.
- Sensitive Products: Even if you're not packaging food or beverages, certain products can be sensitive to potential contamination. For instance, pharmaceuticals and personal care products that come into contact with skin or are ingested should also use low migration inks to avoid any risk of contamination.
- Regulated Markets: If you're exporting products to markets with stringent packaging regulations, such as the European Union or North America, using low migration inks will help ensure compliance. Different regions have varying standards for packaging materials, and low migration inks are a reliable way to meet these diverse requirements.
- Brand Integrity: For brands that prioritize quality and consumer trust, using low migration inks is a proactive measure that underscores a commitment to product safety. This can be a significant differentiator in competitive markets, as consumers increasingly seek brands that prioritize their health and safety.
DOWNLOAD
Find the right low migration ink and coating product for your application
Download Product Selector
Best Practices for Using Low Migration Inks for Rigid Packaging
- Choose the Right Ink Supplier: Partner with suppliers who specialize in low migration inks and have a strong track record in meeting regulatory requirements. Ensure that the inks are certified and tested for low migration by the supplier. INX International validated the low migration properties of INXhrc RC LM natural-based inks by sending printed cups to SQTS (Swiss Quality Testing Services) for Overall migration and Specific migration analysis. Limits are 10mg/dm2 and 60mg/kg food according to Regulation (EU) No 10/2011 and the Swiss Regulation on Food Contact Materials.
- Verify Compliance of printed piece: Responsibility for compliance of the packaging does not lie with one single individual member of the packaging supply chain. It is the ownership of all stakeholders, including the package designer, printer/converter, and material suppliers.
Ultimate responsibility for ensuring compliance rests with the company placing the packaging in the market. To meet target compliance requirements migration studies on the final packaging construction should be performed by an accredited independent laboratory. Depending on risk assessment, ongoing migration testing should be performed to ensure compliance over time in accordance with current industry and government requirements.
What level of migration is acceptable? | ||
|---|---|---|
| The maximum allowable level of migration is based on the toxicological profile of the migrating substance, government regulations for said substance, and brand owner requirements. In every case, the migration profile must first be indentified in order to carry out a risk assessment. | ||
| < 10 ppb | 10 – 50 ppb | > 50 ppb |
No Effect Level Required for toxicologically unevaluated substances or Substances where not enough data exists to judge toxicity. | Evaluate test result Acceptable for substances for which three mutagenicity tests exist and are all negative (i.e. absence of genotoxicity). | Full evaluation needed At this level of migration, the full toxicological profile must be evaluated. The migrant may be an approved additive or otherwise non-toxic. This decision must be made by a competent regulatory and scientific person(s). |
- Understand Packaging Material Compatibility: Low migration inks must be compatible with the specific type of rigid packaging material you're using. For example, INXhrc RC LM is formulated to print on non-porous substrates such as polyester, polystyrene, polyethylene, polypropylene, Styrofoam and polylactic acid (PLA). You should work with your ink supplier to select the right formulation for your needs.
Conclusion
Using low migration inks is not just a matter of compliance; it’s also a commitment to consumer safety and brand integrity. By ensuring that harmful substances do not leach into products, brands can enhance their reputation and build trust with consumers. Furthermore, employing these inks in packaging for sensitive products, including pharmaceuticals and personal care items, is equally important.
To maximize the benefits of low migration inks, it's essential to follow best practices: choose reliable suppliers, verify compliance across the packaging supply chain, and ensure compatibility with specific packaging materials. By doing so, companies can navigate the complexities of packaging regulations while prioritizing consumer health and product quality. In an ever-evolving market, embracing low migration inks is a proactive step toward securing a safer, more responsible future in packaging.
LINK
Safe, Sustainable, Stunning Rigid Containers made possible with INXhrc RC
See if INXhrc RC is <br/> right for you









![Shrink Labels and Recycling [Shrink labellled bottles]](/sites/default/files/styles/large/public/2024-04/INX_shrink_blog850x360.jpg?itok=Qsx6qEqs)






